To serve for CDIBP’s Internationalization Strategy and R&D Innovation Strategy, with open and honest attitude, CDIBP welcome all types of collaboration.
One successful international cooperation case:
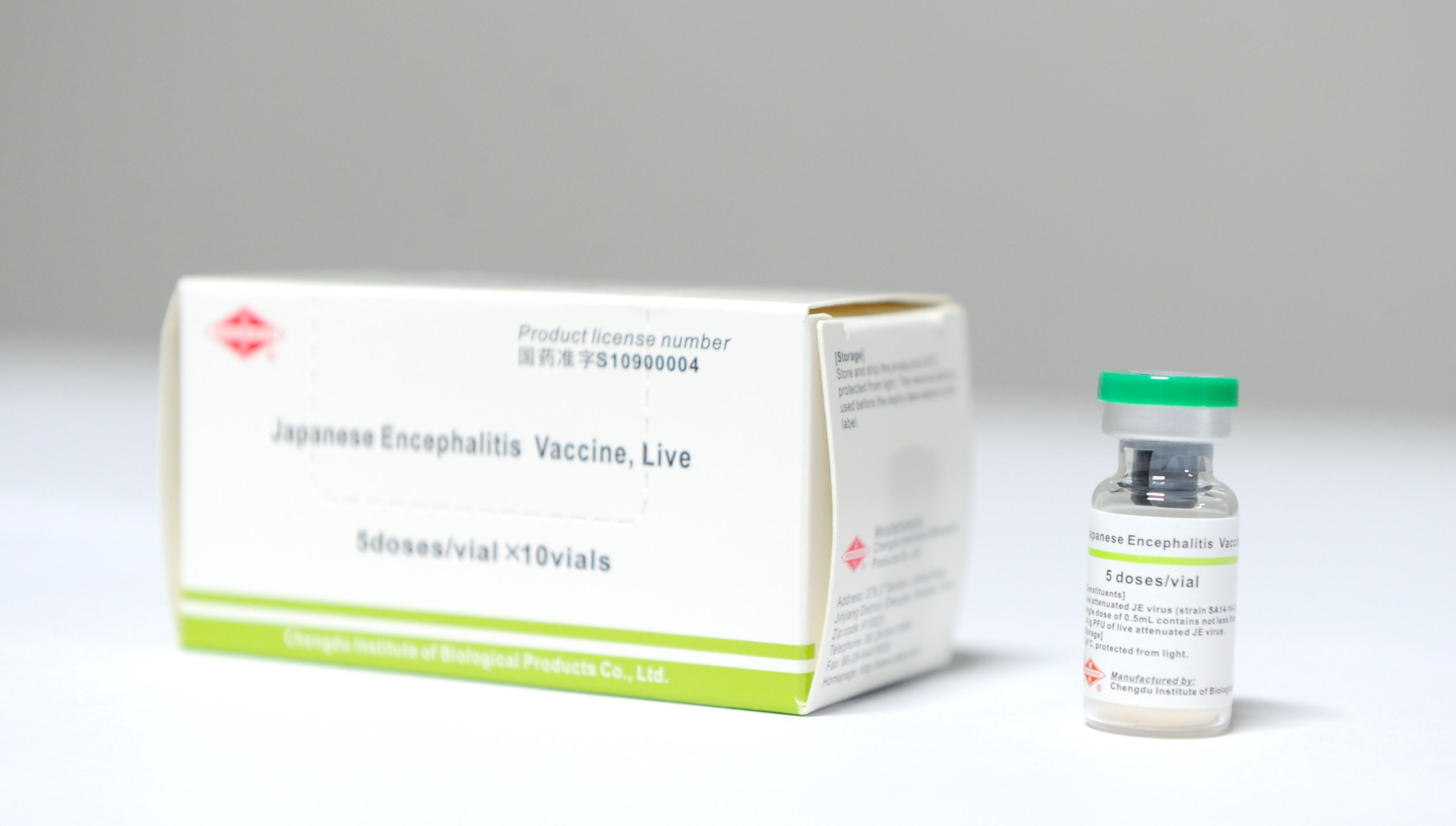
CDIBP cooperated with PATH JE project for WHO prequalification of its Japanese encephalitis vaccine, live (live JE vaccine) , to expand production capacity and improve quality management, achieved the WHO prequalification of live JE vaccine in October 2013. It’s the first vaccine in China to pass WHO prequalification, and can be supplied to United Nations organizations. It opened the road to WHO prequalification of Chinese vaccines. After the vaccine was prequalified by the WHO, it was included in the list of supported vaccines by the Gavi in November 2013, won the bid in 2014 and signed a long-term agreement with UNICEF for supply. The cooperation is a successful case of cooperation between a Chinese enterprise and several international organizations. By 2023, the vaccine has been registered in 13 countries, a total export of more than 530 million doses, making a great contribution to the control of Japanese encephalitis disease in endemic countries.

